(Reuters) -Johnson & Johnson said on Thursday the U.S. Food and Drug Administration had approved its antibody-based therapy for patients with a difficult-to-treat type of blood cancer.
The therapy, talquetamab-tgvs branded as Talvey, belongs to a class of treatments called bispecific antibodies designed to bring a cancer cell and an immune cell together so the body's immune system can kill the cancer.
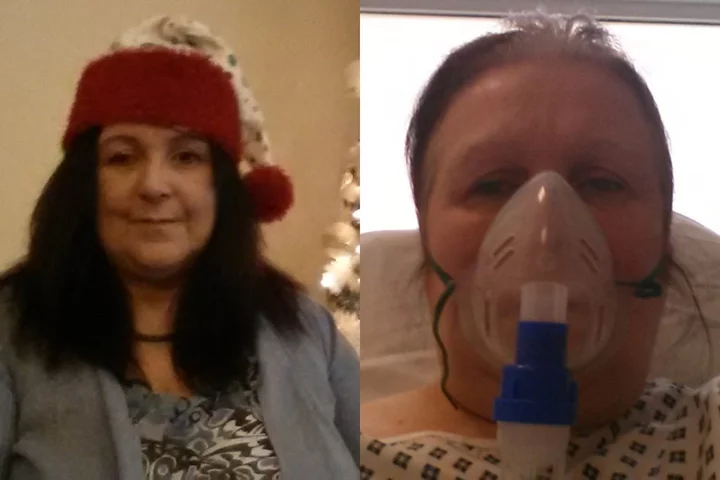
Talvey was approved as a weekly or biweekly subcutaneous, or under-the-skin, injection to treat patients with relapsed multiple myeloma who have received at least four prior lines of treatment, the company said.
Multiple myeloma is a form of cancer that starts in the bone marrow and ultimately disrupts the production of normal blood cells.
Other treatment options available for multiple myeloma include J&J 's Carvykti and Bristol-Myers Squibb's Abecma which belong to a class called CAR-T therapies, or chimeric antigen receptor T-cell therapies. They work by harvesting a patient's own disease-fighting T-cells, genetically engineering them to target specific proteins on cancer cells, and replacing them to seek out and attack cancer.
"Although options for the treatment of multiple myeloma have expanded significantly in recent years, the disease remains incurable, and therefore, patients are in need of new treatment options," said Michael Andreini, CEO of the non-profit Multiple Myeloma Research Foundation.
The FDA also placed its strongest "boxed" safety warning on the drug's label, flagging the risk for a type of aggressive immune response or cytokine release syndrome and neurologic toxicity.
The accelerated approval is based on data from a mid-stage study, which showed 73.6% patients achieved either partial or complete disappearance of cancer from their body.
(Reporting by Mariam Sunny in Bengaluru; Editing by Krishna Chandra Eluri and Maju Samuel)