Covid-19 shots should be reformulated to target an XBB subvariant of the highly infectious omicron strain to provide the best protection during the US fall and winter season, advisers to health regulators said.
Vaccines made by the partnership of Pfizer Inc. and BioNTech SE, Moderna Inc. and Novavax Inc. should be updated to protect against XBB strains, advisers to the US Food and Drug Administration said Thursday in a unanimous vote. The agency isn’t required to follow the panel’s recommendations, but often does.
“We need a better vaccine,” said Eric Rubin, a panel member and professor at the Harvard T.H. Chan School of Public Health. “We should be updating it, I think it’s pretty straightforward.”
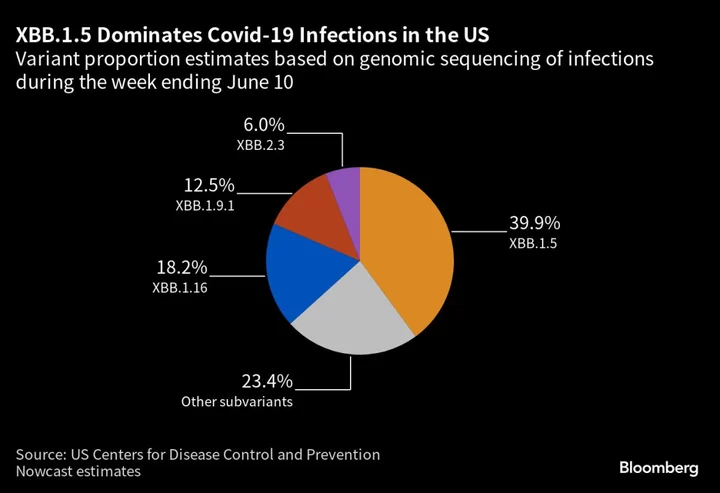
The experts mostly agreed that the XBB.1.5 subvariant appears to be the best option for future vaccines, given early studies have shown it’s able to provide cross-protection against other XBB subvariants, such as XBB.1.16 and XBB.2.3.
“We in general feel that XBB.1.5 would be preferred,” said committee chair Arnold Monto, a professor of epidemiology at the University of Michigan. “The fact that most of the manufacturers are ready to work on XBB.1.5 is an added reason to select this variant.”
The XBB.1.5 strain currently accounts for about 40% of Covid infections in the US, according to the Centers for Disease Control and Prevention. The strain selection process mirrors how recommendations are made for the influenza vaccine each year based on which flu strains are dominant. The goal is to have updated Covid shots ready in September. The question remains whether Americans will actually get them, with just 17% of those eligible having received the bivalent BA.4 and BA.5 booster.
The vote signaled a potential return to vaccines that only use a single strain of the virus, like the earliest shots that targeted only the original strain first seen in Wuhan, China. Vaccine makers assessed several different candidate vaccines in preparation for the meeting, but vaccines using only XBB.1.5 appeared to provide the best protection against currently circulating variants in both animal and human studies, the data show.
“The data support that variant-adapted vaccines improve protection,” said Kena Swanson, head of viral vaccine research and development at Pfizer. “An updated vaccine more closely matched to the circulating XBBs is warranted.”
Pfizer, Moderna and Novavax said in the meeting that updated vaccines could be developed and manufactured in time for a rollout in the US fall, assuming that regulators at the FDA issue a final decision about strain composition in the coming days. Novavax said that its production would be delayed if a strain other than XBB.1.5 is recommended.
Improving Durability
The companies also assessed safety for the updated vaccine candidates, which was similar to previous versions of the shots.
Last summer, the FDA asked vaccine makers to reformulate mRNA-based shots to add omicron subvariants BA.4 and BA.5, which were dominant at the time. Subsequent studies have shown that the boosters provided some modest, short-term benefit, mostly for older adults.
Improving the durability of vaccines remains an ongoing challenge that needs to be addressed, Jerry Weir, director of the FDA’s division of viral products, said during the meeting.
Thursday’s vote helps align the US with other global public health agencies, after the World Health Organization and European Medicines Agency both said that evidence supports using a vaccine based on a single strain of XBB, such as XBB.1.5 or XBB.1.16. Currently available vaccines continue to provide good protection against severe disease, but data suggest updating shots to target newer variants will help broaden immunity against currently circulating variants and any others they spawn, WHO experts have said.
Health officials have largely stopped tracking Covid cases in real time, but hospitalizations and deaths remain at their lowest levels since the pandemic began, largely due to widespread vaccination. About 75% of vaccinated Americans who have developed severe Covid symptoms have also had multiple risk factors, like older age and underlying medical conditions, Natalie Thornburg, an expert in the CDC’s coronavirus and respiratory viruses division, said in the VRBPAC meeting.
Read More: US Covid Case Count to Go Dark as Public Health Emergency Halted
(Updates with strain selection discussion from fourth paragraph.)